Benchmarking practices and research
(Health Data Warehouse transparency portal)
![]()
Join a research program
In partnership with hospitals, LogipremF has established a benchmarking program for care practices. The use of Logipren prescription software is coupled with the establishment of a centralized prescription database (Logipren Database). These data are pseudonymised (for the patient and the prescriber) within the facilities, before being sent to the Health Data Warehouse (HDW).
The primary objective is to provide neonatology and pediatrics services with comparative results of their prescription practices (benchmarking program).
The secondary objective is to provide research units with a real-world prescription database for medical studies (see ongoing research and publications below).
A Scientific and Ethical Committee has been established, comprised notably of physicians and pharmacists from all user care services, representatives of learned societies (SFN, SFPC), and research units (CEPOI, INSERM), for the management of the constituted database.
This Scientific Committee meets at least once a year and guides research using the Logipren Database.
![]()
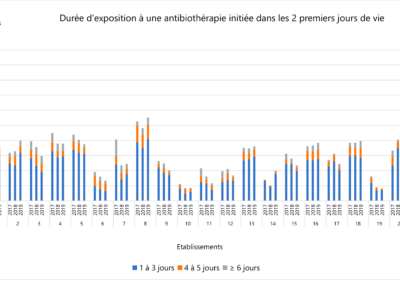
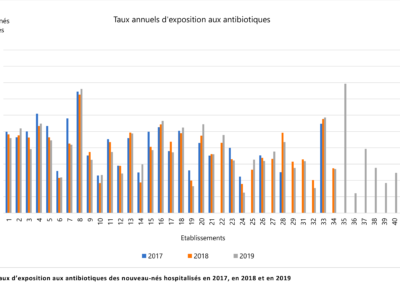
Comparison of prescription practices
The LOGIPREN database allows for regular comparison of prescription practices between care units.
Each year, a report is drafted containing the results of each unit, as well as graphs comparing these results.
The comparison is anonymous: each care unit is associated with a number used for the presented results.
Everyone knows their own number, but not those of others. The results are analyzed annually in meetings by physicians and pharmacists from all hospitals.

![]()
Ongoing studies
Prescription modalities of iron in preterm infants <32 weeks of gestational age and <1500 g: Mathis Thia, Silvia Iacobelli (CEPOI), Elsa Kermorvant (Necker)
Mupirocin: Isabelle Ligi (APHM, University Hospital La Conception)
Sodium intake in the newborn: JM Hascoët (CHU Nancy)
Prediction of patient risks in the NICU: E Jolivet, Y Lucas (Logipren)
Feel free to contact us to participate or propose study topics.
![]()
Scientific articles
Vaccination of Very Preterm Infants in French Neonatal Units
Acta Paediatrica 2025, SPECIAL ISSUE Childhood Vaccinations Now and in the Future
Charkaluk M.-L., Gouyon B., Simon Lorrain S., Nuytten A.
Electronic versus handwritten medication prescriptions improves safety in neonatal intensive care units
Drugs & Therapy Perspectives, 2025
Hascoët, J.-M., Gouyon, B., Decobert, F., Escourrou, G., Boileau, P., Boize, P., Hamon, I., Mitanchez, D., Gobalakichenane, P., Robine, A., Ghostine, G., Juguera Rodriguez, L., Pellicer, A., Gouyon, J.-B.
Estimated equipotent conversion ratios of morphine, sufentanil and fentanyl as continuous infusion in neonatal intensive care units: a pharmacoepidemiologic cohort study
European Journal of Clinical Pharmacology, 2025
Tauzin, M., Cavalier, I., Ortala, M., Jung, C., Gouyon, B., Durrmeyer, X.,
Prescriptions of anti-reflux drugs in neonatology and neonatal intensive care units: A large multicentre observational study
(2014–2022)
British Journal of Clinical Pharmacology, 2024
Tauzin, M., Gouyon, B., Jiao, L., Lapillonne, A., Lorrain, S., Bellaiche, M., Jung, C.(2024).
Use of dexmedetomidine during mechanical ventilation in extremely preterm and extremely low birth weight neonates receiving morphine: A single-center retrospective study
Paediatric and Neonatal Pain, 2024
Camille Irving, Xavier Durrmeyer, Fabrice Decobert, Gilles Dassieu, Aroua Ben Guirat, Béatrice Gouyon, Manon Tauzin (2024).
Diuretic drug utilization in neonates: a French prescription database analysis
Frontiers in Pharmacology, 2024
Lacobelli,S., Lorrain, S., Rabe, E., Gouyon, B., Gouyon, J.B., & Bonsante, F. (2024).
Diuretic drug utilization in neonates: a French prescription database analysis, 15:1358761.
Postnatal corticosteroid exposure in very preterm infants : A french cohort study
Frontiers in Pharmacology, 2023
Iacobelli, S., Allamèle-Moutama, K., Lorrain, S., Gouyon, B., Gouyon, J. B., Bonsante, F., & Logipren Collaborative Working Group (2023).
Postnatal corticosteroid exposure in very preterm infants: A French cohort study. Frontiers in pharmacology, 14, 1170842.
Paraben exposure through drugs in the neonatal intensive care unit : a regional cohort study
Frontiers in Pharmacology, 2023
Iacobelli, S., Commins, M., Lorrain, S., Gouyon, B., Ramful, D., Richard, M., Grondin, A., Gouyon, J. B., & Bonsante, F. (2023).
Paraben exposure through drugs in the neonatal intensive care unit: a regional cohort study. Frontiers in pharmacology, 14, 1200521.
Frequencies, Modalities, Doses and Duration of Computerized Prescriptions for Sedative, Analgesic, Anesthetic and Paralytic Drugs in Neonates Requiring Intensive Care: A Prospective Pharmacoepidemiologic Cohort Study in 30 French NICUs From 2014 to 2020
Frontiers in Pharmacology, 2022
Tauzin, M., Gouyon, B., Hirt, D., Carbajal, R., Gouyon, J. B., Brunet, A. C., Ortala, M., Goro, S., Jung, C., & Durrmeyer, X. (2022).
Frequencies, Modalities, Doses and Duration of Computerized Prescriptions for Sedative, Analgesic, Anesthetic and Paralytic Drugs in Neonates Requiring Intensive Care : A Prospective Pharmacoepidemiologic Cohort Study in 30 French NICUs From 2014 to 2020. Frontiers in pharmacology, 13, 939869.
Copy the URL to download : https://www.logipren.com/wp-content/uploads/2022/07/2022.07.18_Article-antalgiques-M-Tauzin.pdf
Prescription of Aminoglycosides in 23 French Neonatal Intensive Care Units
Antibiotics, 2021
Martin-Mons, S., Gouyon, B., Lorrain, S., Abasse, S., Alexandre, C., Binson, G., Brat, R., Caeymaex, L., Couringa, Y., Desbruyeres, C., Meglio, M. D., Escourrou, G., Flamein, F., Flechelles, O., Girard, O., Kermorvant-Duchemin, E., Lapillonne, A., Lafon, C., Di Maio, M., Mazeiras, G., … Gouyon, J. B. (2021).
Prescription of Aminoglycosides in 23 French Neonatal Intensive Care Units. Antibiotics (Basel, Switzerland), 10(11), 1422.
Copy the URL to download : https://www.logipren.com/wp-content/uploads/2022/07/2021.11.21_Prescription-of-Aminoglycosides-in-23-French-Neonatal-Intensive-Care-units.pdf
Antibiotics prescription over three years in a French benchmarking network of 23 Level 3 Neonatal Wards
Frontiers in Pharmacology, 2020
Martin-Mons, S., Lorrain, S., Iacobelli, S., Gouyon, B., Gouyon, J. B., & B-PEN Study Group (2021).
Antibiotics Prescription Over Three Years in a French Benchmarking Network of 23 Level 3 Neonatal Wards. Frontiers in pharmacology, 11, 585018.
https://www.frontiersin.org/articles/10.3389/fphar.2020.585018/abstract
https://doi.org/10.3389/fphar.2020.585018
Drug exposure for PDA closure in France: a prospective, cohort-based, analysis
European journal of clinical pharmacology.2020
Iacobelli, S., Lorrain, S., Gouyon, B., Gambacorta, S., Laforgia, N., Gouyon, J. B., & Bonsante, F. (2020).
Drug exposure for PDA closure in France: a prospective, cohort-based, analysis. European journal of clinical pharmacology, 76(12), 1765–1772.
Characteristics of prescription in 29 Level 3 Neonatal Wards over a 2-year period (2017-2018). An inventory for future research
Public Library of Science one (Plos One),2019
Gouyon, B., Martin-Mons, S., Iacobelli, S., Razafimahefa, H., Kermorvant-Duchemin, E., Brat, R., Caeymaex, L., Couringa, Y., Alexandre, C., Lafon, C., Ramful, D., Bonsante, F., Binson, G., Flamein, F., Moussy-Durandy, A., Di Maio, M., Mazeiras, G., Girard, O., Desbruyeres, C., Mourdie, J., … Gouyon, J. B. (2019).
Characteristics of prescription in 29 Level 3 Neonatal Wards over a 2-year period (2017-2018). An inventory for future research. PloS one, 14(9), e0222667.
A Computer Prescribing Order Entry-Clinical Decision Support system designed for neonatal care: results of the 'preselected prescription' concept at the bedside
Journal of clinical pharmacy and therapeutics,2017
Gouyon, B., Iacobelli, S., Saliba, E., Quantin, C., Pignolet, A., Jacqz-Aigrain, E., & Gouyon, J. B. (2017).
A Computer Prescribing Order Entry-Clinical Decision Support system designed for neonatal care: results of the 'preselected prescription' concept at the bedside. Journal of clinical pharmacy and therapeutics, 42(1), 64–68.
![]()
Data protection
Data security is at the heart of our concerns.
Our teams have implemented advanced measures to ensure the confidentiality and integrity of the information we process.
All data processing related to the database is in strict compliance with current regulations:
- The entire processing is compliant with the European General Data Protection Regulation (GDPR)
- The evaluation of care practices is authorised by the National Commission on Informatics and Liberty (historically: DE-2017-410 then MR004, Deliberation No. 2018-155 of 3rd May 2018).
- The collection, storage, and availability of benchmarking data for other research projects are subject to CNIL authorization for the Logipren Health Data Warehouse (DATE DT-2024-013).
- The reuse of EDS Logipren data in another medical research project falls within the MR004 framework.
For more information on Health Data Warehouses in France : https://www.cnil.fr/fr/explorez-la-cartographie-des-entrepots-de-donnees-de-sante-en-france
At any time, upon request, a patient’s data can be removed from the Logipren database.
To learn more (about your rights and our processing of health data), please consult our privacy policy:
SAS LogipremF Paris Branch
104 boulevard Auguste Blanqui
75013 Paris
SAS LogipremF Headquarters
27, Avenue du Dr Jean Marie Dambreville, Terre Sainte
97410 Saint-Pierre - La Réunion